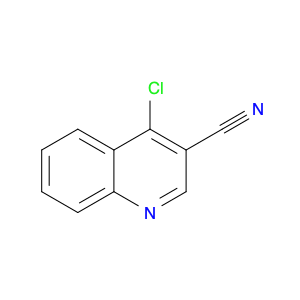
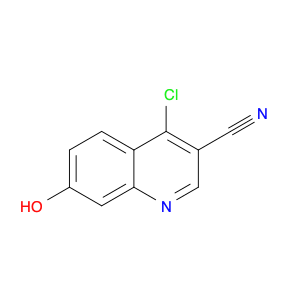
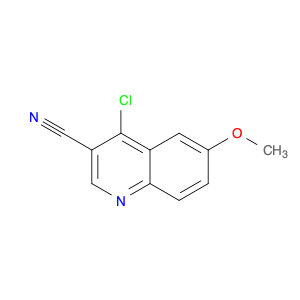
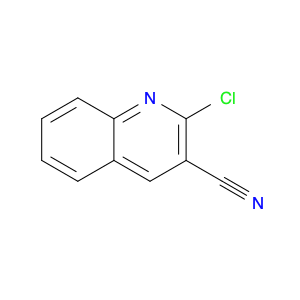
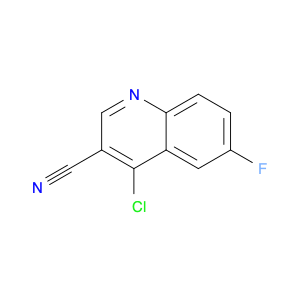
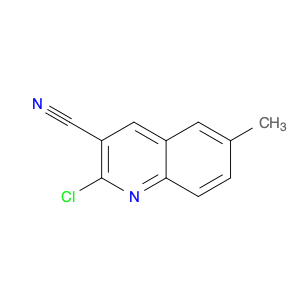
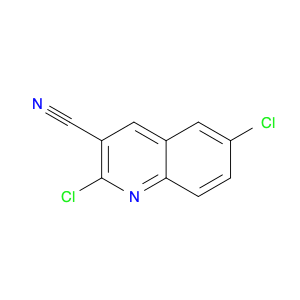
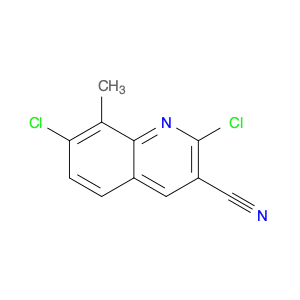
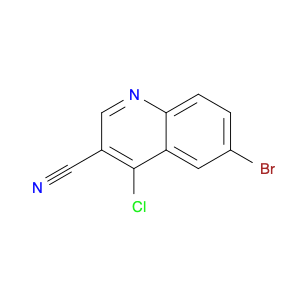
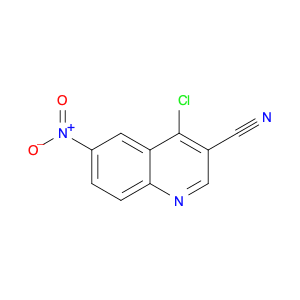
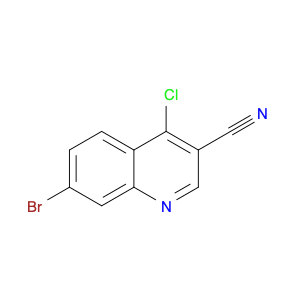
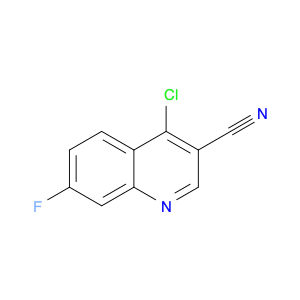
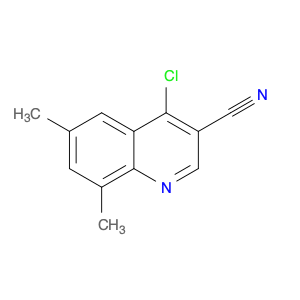
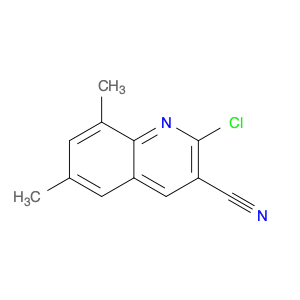
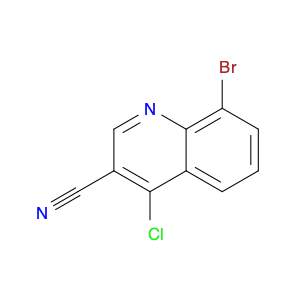
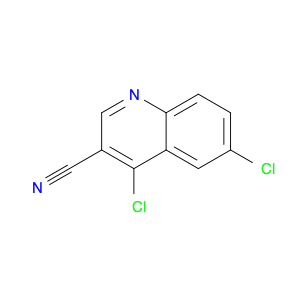
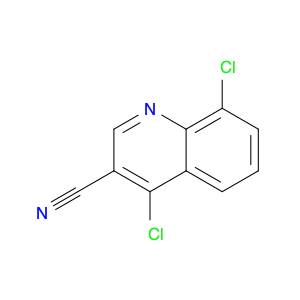
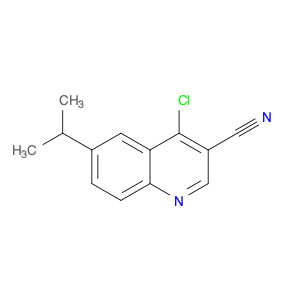
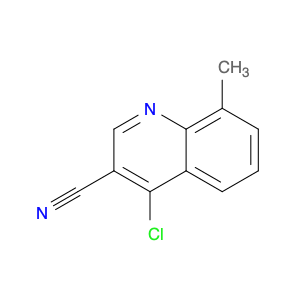
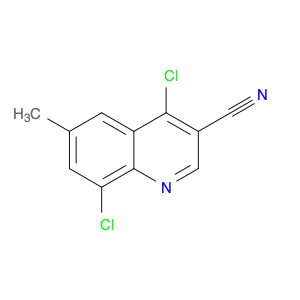
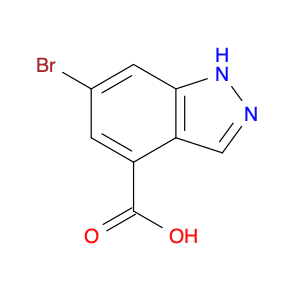
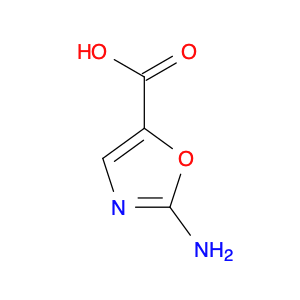
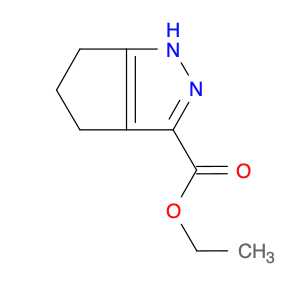
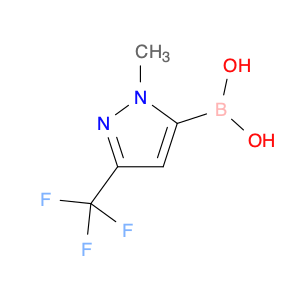
4-Chloroquinoline-3-carbonitrile, also known by its chemical formula C10H5ClN2, serves as a versatile building block in chemical synthesis. This compound finds wide application in the pharmaceutical and agrochemical industries due to its unique chemical properties.One key application of 4-Chloroquinoline-3-carbonitrile is in the synthesis of various bioactive molecules, such as pharmaceutical drugs and agricultural chemicals. By acting as a precursor in the production of more complex compounds, it enables the creation of novel chemical entities with diverse biological activities.In organic synthesis, 4-Chloroquinoline-3-carbonitrile can undergo a variety of reactions to introduce functional groups at specific positions on the quinoline ring. For instance, it can participate in nucleophilic substitution reactions, palladium-catalyzed cross-coupling reactions, and aromatic substitution reactions, allowing for the tailored modification of its structure.Furthermore, its nitrile functional group provides a handle for further derivatization, enabling the attachment of additional chemical moieties for enhancing the target molecule's pharmacological or pesticidal properties. The presence of the chlorine atom in the 4-position confers specific reactivity and electronic properties to the molecule, further expanding its synthetic utility.Overall, 4-Chloroquinoline-3-carbonitrile serves as a valuable intermediate in chemical synthesis, playing a crucial role in the development of new compounds for potential therapeutic and agricultural applications.
 sales@aaronchem.com
sales@aaronchem.com