Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
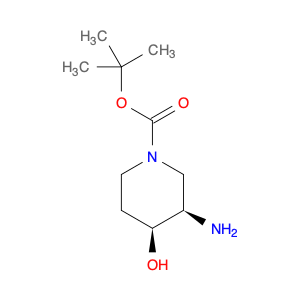
1-Piperidinecarboxylic acid, 3-amino-4-hydroxy-, 1,1-dimethylethyl ester, (3R,4S)-
Catalog#: AR000WJS | CAS#: 1312798-50-7 | MDL#: MFCD28502771 | MF: C10H20N2O3 | MW: 216.2774
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 50mg | $78.00 | Global Stock | Buy Now | Add To Cart | ||
| 100mg | $87.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $139.00 | Global Stock | Buy Now | Add To Cart | ||
| 500mg | $174.00 | Global Stock | Buy Now | Add To Cart | ||
| 1g | $295.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $1,247.00 | Global Stock | Buy Now | Add To Cart | ||
| 10g | $2,164.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
| Catalog Number | AR000WJS |
| Chemical Name | 1-Piperidinecarboxylic acid, 3-amino-4-hydroxy-, 1,1-dimethylethyl ester, (3R,4S)- |
| CAS Number | 1312798-50-7 |
| Molecular Formula | C10H20N2O3 |
| Molecular Weight | 216.2774 |
| MDL Number | MFCD28502771 |
| SMILES | O[C@H]1CCN(C[C@H]1N)C(=O)OC(C)(C)C |