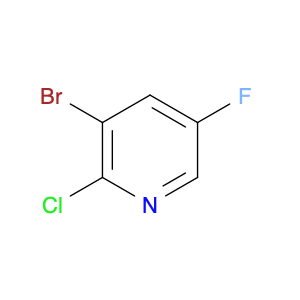
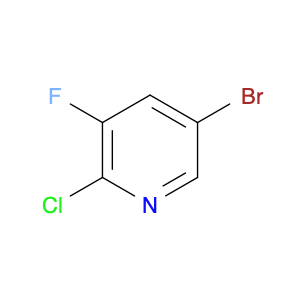
3-Bromo-2-chloro-5-fluoropyridine, a versatile chemical compound, serves as a valuable building block in chemical synthesis due to its unique reactivity and structural properties. In the field of organic chemistry, this compound can be utilized as a key intermediate in the synthesis of complex molecules and pharmaceuticals. Its trifunctional nature, incorporating bromine, chlorine, and fluorine substituents on the pyridine ring, enables diverse derivatization strategies, allowing for the introduction of additional functional groups and modifications.One of the primary applications of 3-Bromo-2-chloro-5-fluoropyridine is its role in the development of agrochemicals and pharmaceuticals. By serving as a precursor in the synthesis of biologically active molecules, this compound plays a crucial part in drug discovery and development processes. Its selective halogenation pattern provides a specific site for further elaboration, facilitating the construction of molecular scaffolds with enhanced biological activity and target specificity.Moreover, 3-Bromo-2-chloro-5-fluoropyridine can participate in cross-coupling reactions, such as Suzuki, Heck, or Stille couplings, leading to the formation of carbon-carbon or carbon-heteroatom bonds. These transformations enable the construction of complex molecular architectures and the incorporation of functional groups necessary for the desired chemical properties.Overall, the strategic position of bromine, chlorine, and fluorine moieties in 3-Bromo-2-chloro-5-fluoropyridine makes it a valuable tool in chemical synthesis, allowing for the creation of structurally diverse compounds with potential applications in medicinal chemistry, materials science, and agrochemical research.
 sales@aaronchem.com
sales@aaronchem.com