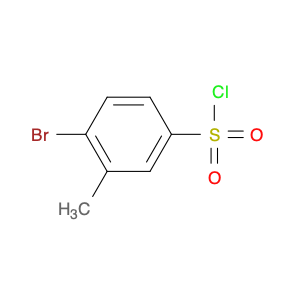
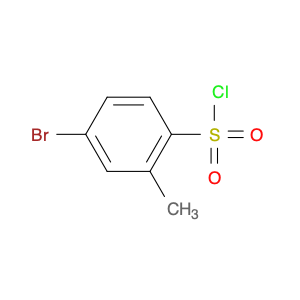
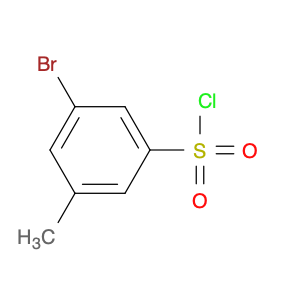
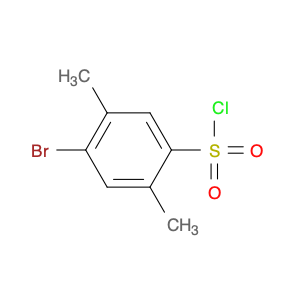
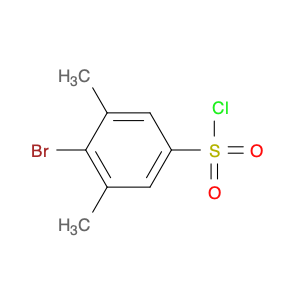
4-Bromo-3-methylbenzene-1-sulfonyl chloride, also known as Bromo-m-toluenesulfonyl chloride, is a versatile reagent widely used in chemical synthesis. This compound serves as a valuable chlorosulfonylation reagent in organic reactions due to its unique properties and reactivity. In chemical synthesis, 4-Bromo-3-methylbenzene-1-sulfonyl chloride can be employed in various transformations to introduce sulfonyl chloride functionality into organic molecules. Its reactivity as a sulfonyl chloride derivative allows for the formation of robust carbon-sulfur bonds, which are crucial for the modification and functionalization of organic compounds. One common application of 4-Bromo-3-methylbenzene-1-sulfonyl chloride is in the synthesis of sulfonamide derivatives, where it serves as a key intermediate for the introduction of sulfonyl groups onto nitrogen-containing compounds. This reaction pathway is essential in the pharmaceutical industry for the production of diverse bioactive molecules with potential therapeutic properties. Additionally, 4-Bromo-3-methylbenzene-1-sulfonyl chloride can also be used in the preparation of sulfonate esters, sulfonamides, and other functionalized organic molecules through various coupling reactions and transformations. Its compatibility with a wide range of nucleophiles and reaction conditions makes it a valuable tool for chemists seeking to modify and manipulate organic structures for research and industrial purposes.
 sales@aaronchem.com
sales@aaronchem.com