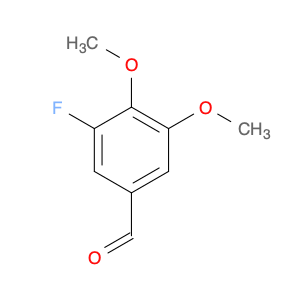
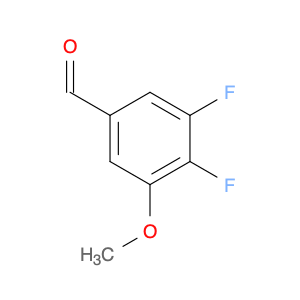
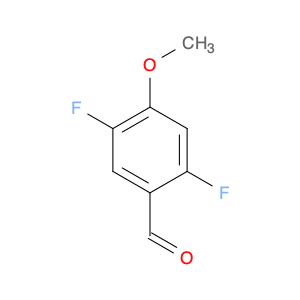
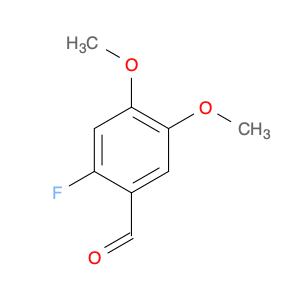
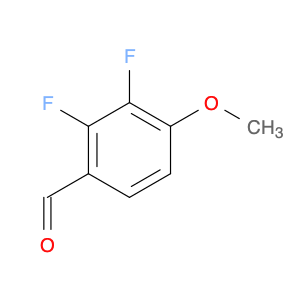
3-Fluoro-4,5-dimethoxybenzaldehyde, also known as $name$, is a versatile compound widely used in chemical synthesis. Its primary application lies in organic synthesis, where it serves as a key building block in the creation of various pharmaceuticals, agrochemicals, and advanced materials.In chemical synthesis, $name$ is valued for its unique molecular structure, which allows it to participate in a wide range of reactions to form complex organic molecules. Its fluorine and methoxy functional groups provide opportunities for selective modifications, making it a valuable tool for the strategic design of new compounds.One of the key reactions involving $name$ is the Vilsmeier-Haack formylation, where it can be used as a precursor for introducing formyl groups into aromatic systems. This reaction is commonly employed in the synthesis of pharmaceutical intermediates and fine chemicals.Additionally, $name$ can participate in various cross-coupling reactions, such as Suzuki-Miyaura or Buchwald-Hartwig reactions, enabling the creation of biaryl compounds with enhanced pharmacological properties or materials with specific electronic properties.Furthermore, the fluoro substituent in $name$ can impart unique physicochemical properties to the resulting molecules, such as improved metabolic stability or enhanced binding affinity to biological targets. This feature makes $name$ a valuable tool in drug discovery and development efforts.Overall, the versatile nature of 3-Fluoro-4,5-dimethoxybenzaldehyde makes it an essential component in the toolkit of synthetic chemists, offering a wide array of possibilities for the construction of diverse and valuable organic molecules.
 sales@aaronchem.com
sales@aaronchem.com