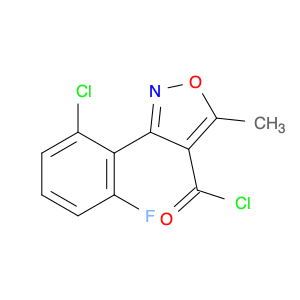
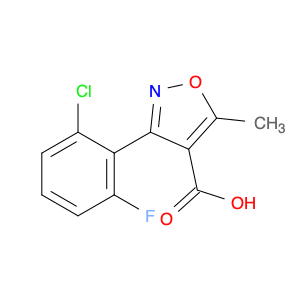
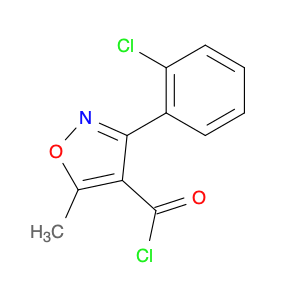
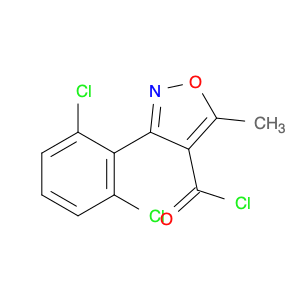
The compound 3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonyl chloride, typically referred to as $name$, has found significant utility in chemical synthesis processes. Utilizing its carbonyl chloride functional group, this compound serves as a versatile building block in the creation of various organic compounds.1. **Acylation Reactions**: One of the notable applications of $name$ is in acylation reactions, where the carbonyl chloride moiety acts as an acylating agent. By reacting with nucleophiles under appropriate conditions, $name$ can facilitate the introduction of the isoxazole-4-carbonyl group into diverse molecules, enabling the synthesis of complex organic compounds.2. **Medicinal Chemistry**: In the realm of medicinal chemistry, 3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonyl chloride is often employed for the design and development of pharmaceutical agents. Through its reactivity and ability to modify molecular structures, this compound plays a crucial role in the creation of new drug candidates with potential therapeutic applications.3. **Functional Group Transformation**: The presence of the carbonyl chloride functionality in $name$ allows for the selective transformation of functional groups in target molecules. This attribute is particularly valuable in multistep synthesis pathways, where precise derivatization and modification of organic compounds are essential for achieving the desired chemical structures.In summary, the strategic incorporation of 3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonyl chloride in chemical synthesis workflows underscores its importance as a key reagent for generating diverse organic compounds with tailored properties and functionalities.
 sales@aaronchem.com
sales@aaronchem.com