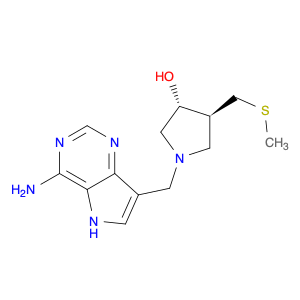
The compound (3R,4S)-1-[(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)methyl]-4-[(methylthio)methyl]-3-pyrrolidinol is a valuable tool in chemical synthesis due to its unique structural properties and reactivity. This compound serves as a versatile building block in organic synthesis, particularly in the formation of complex molecules for pharmaceuticals, agrochemicals, and materials science.One key application of (3R,4S)-1-[(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)methyl]-4-[(methylthio)methyl]-3-pyrrolidinol is its role as a chiral auxiliary in asymmetric synthesis. The presence of chiral centers in the compound allows for the stereocontrolled construction of molecules with high enantioselectivity. By incorporating this compound into synthetic pathways, chemists can access enantiomerically pure compounds that are crucial in drug discovery and development.Additionally, the amino and thiol functional groups present in (3R,4S)-1-[(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)methyl]-4-[(methylthio)methyl]-3-pyrrolidinol enable a wide range of chemical transformations, including nucleophilic substitution reactions, nucleophilic additions, and various coupling reactions. This versatility makes the compound a valuable tool for the construction of diverse molecular scaffolds with tailored properties.In conclusion, the strategic incorporation of (3R,4S)-1-[(4-Amino-5H-pyrrolo[3,2-d]pyrimidin-7-yl)methyl]-4-[(methylthio)methyl]-3-pyrrolidinol in chemical synthesis enables the synthesis of complex molecules with precise stereochemistry and functional group compatibility. Its applications extend to various branches of chemistry, offering opportunities for innovative synthetic routes and the creation of novel compounds with potential applications across industries.
 sales@aaronchem.com
sales@aaronchem.com