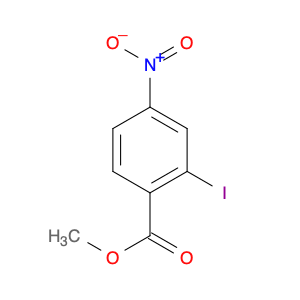
Methyl 2-iodo-4-nitrobenzoate, a key compound in organic chemistry, plays a crucial role in various chemical synthesis processes. Primarily, this chemical is utilized as a versatile building block in the creation of complex molecular structures. Its unique molecular structure, characterized by the presence of an ester functional group, an iodine atom, and a nitro group, makes it an essential reagent in the synthesis of pharmaceuticals, agrochemicals, and advanced materials.In organic synthesis, Methyl 2-iodo-4-nitrobenzoate acts as a valuable precursor for the introduction of the iodo substituent into target molecules. This iodo group serves as a potent directing group in cross-coupling reactions, such as Suzuki-Miyaura and Heck reactions, facilitating the formation of carbon-carbon or carbon-heteroatom bonds. Furthermore, the nitro group in the compound can be selectively reduced to an amino group, enabling the synthesis of various functionalized aromatic compounds.Additionally, Methyl 2-iodo-4-nitrobenzoate exhibits reactivity towards nucleophiles, allowing for the creation of diverse chemical entities through substitution reactions. Its compatibility with a variety of reaction conditions and its ability to undergo transformations under mild reaction conditions make it a valuable tool for chemists in the development of novel compounds with targeted properties.Overall, Methyl 2-iodo-4-nitrobenzoate serves as a crucial intermediate in organic synthesis, enabling chemists to access a wide range of functionalized molecules with diverse applications in the fields of pharmaceuticals, materials science, and agrochemicals.
 sales@aaronchem.com
sales@aaronchem.com