Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
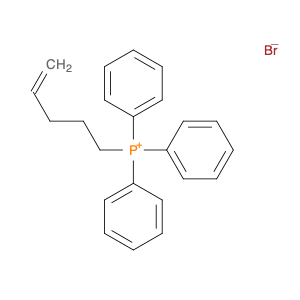
Pent-4-en-1-yltriphenylphosphonium bromide
Catalog#: AR003CLL | CAS#: 56771-29-0 | MDL#: MFCD00050215 | MF: C23H24BrP | MW: 411.3144
| Availability | ||
|---|---|---|
| Typically In Stock |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR003CLL |
| Chemical Name | Pent-4-en-1-yltriphenylphosphonium bromide |
| CAS Number | 56771-29-0 |
| Molecular Formula | C23H24BrP |
| Molecular Weight | 411.3144 |
| MDL Number | MFCD00050215 |
| SMILES | C=CCCC[P+](c1ccccc1)(c1ccccc1)c1ccccc1.[Br-] |

Butanoic acid, 3-[[(1,1-dimethylethoxy)carbonyl]amino]-4-hydroxy-, phenylmethyl ester, (3R)-
182748-72-7

1-Piperidinecarboxylic acid, 4-[[4-(methoxycarbonyl)phenyl]methyl]-, 1,1-dimethylethyl ester
210964-04-8
| GHS Pictogram | N/A |
| UN# | - |
| Hazard Statements | - |
| Class | - |
| Packing Group | - |