Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
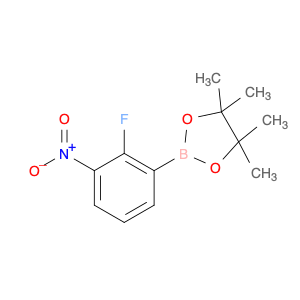
2-(2-Fluoro-3-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Catalog#: AR0075UU | CAS#: 1189042-70-3 | MDL#: MFCD15143593 | MF: C12H15BFNO4 | MW: 267.0612
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 100mg | $5.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $8.00 | Global Stock | Buy Now | Add To Cart | ||
| 1g | $20.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $82.00 | Global Stock | Buy Now | Add To Cart | ||
| 10g | $156.00 | Global Stock | Buy Now | Add To Cart | ||
| 25g | $298.00 | Global Stock | Buy Now | Add To Cart | ||
| 50g | $554.00 | Global Stock | Buy Now | Add To Cart | ||
| 100g | $1,039.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR0075UU |
| Chemical Name | 2-(2-Fluoro-3-nitrophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane |
| CAS Number | 1189042-70-3 |
| Molecular Formula | C12H15BFNO4 |
| Molecular Weight | 267.0612 |
| MDL Number | MFCD15143593 |
| SMILES | CC1(C)OB(OC1(C)C)c1cccc(c1F)[N+](=O)[O-] |
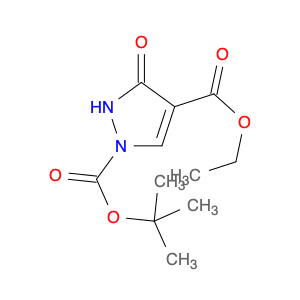
1H-Pyrazole-1,4-dicarboxylic acid, 2,3-dihydro-3-oxo-, 1-(1,1-dimethylethyl) 4-ethyl ester
178424-17-4
| GHS Pictogram |

|
| Signal Word | Warning |
| UN# | - |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305+P351+P338 |
| Class | - |
| Packing Group | - |