Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com

1-Propanaminium, N,N,N-tripropyl-, (T-4)-tetraoxoruthenate(1-) (1:1)
Catalog#: AR000FIO | CAS#: 114615-82-6 | MDL#: MFCD00074914 | MF: C12H28NO4Ru | MW: 351.425
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 100mg | $4.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $5.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR000FIO |
| Chemical Name | 1-Propanaminium, N,N,N-tripropyl-, (T-4)-tetraoxoruthenate(1-) (1:1) |
| CAS Number | 114615-82-6 |
| Molecular Formula | C12H28NO4Ru |
| Molecular Weight | 351.425 |
| MDL Number | MFCD00074914 |
| SMILES | [O-][Ru](=O)(=O)=O.CCC[N+](CCC)(CCC)CCC |
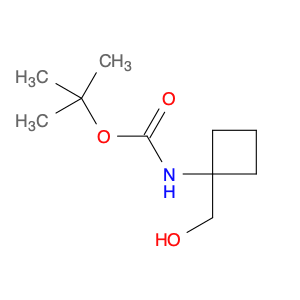
Carbamic acid, N-[1-(hydroxymethyl)cyclobutyl]-, 1,1-dimethylethyl ester
1142211-17-3
| GHS Pictogram |


|
| Signal Word | Danger |
| UN# | 1479 |
| Hazard Statements | H272-H315-H319-H335 |
| Precautionary Statements | P220-P261-P305+P351+P338 |
| Class | 5.1 |
| Packing Group | Ⅱ |