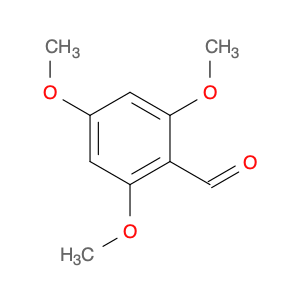
2,4,6-Trimethoxybenzaldehyde, also known as TMB, is a versatile chemical compound widely used in chemical synthesis applications. In organic chemistry, TMB is commonly utilized as a building block for the synthesis of various biologically active molecules and pharmaceuticals. Its unique structure and reactivity make it a valuable precursor in the preparation of complex organic compounds.One of the key applications of 2,4,6-Trimethoxybenzaldehyde is in the synthesis of heterocyclic compounds. By reacting TMB with different reagents and catalysts, chemists can efficiently construct a variety of heterocycles such as pyrroles, pyrazoles, and indoles. These heterocyclic compounds are essential components in the development of drugs, agrochemicals, and materials with diverse functionalities.Additionally, 2,4,6-Trimethoxybenzaldehyde serves as a crucial intermediate in the production of flavoring agents and fragrances. Its aromatic nature and methoxy groups contribute to the creation of unique scents and tastes when incorporated into perfumes, food additives, and other aromatic products. By manipulating the chemical properties of TMB through various synthetic pathways, chemists can tailor the final fragrance or flavor profile according to specific requirements.Moreover, the presence of three methoxy groups on the benzene ring of TMB provides opportunities for further functionalization reactions. Chemists can selectively modify the methoxy groups to introduce additional chemical functionalities, enhancing the versatility of TMB in organic synthesis. These modifications enable the fine-tuning of physical and chemical properties of the resulting compounds for a wide range of applications in the pharmaceutical, agrochemical, and materials industries.
 sales@aaronchem.com
sales@aaronchem.com