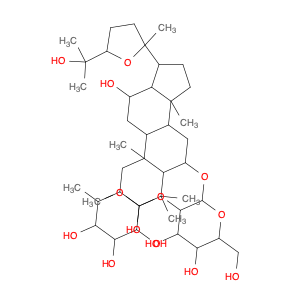
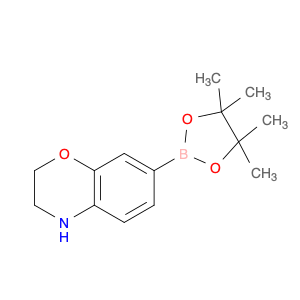
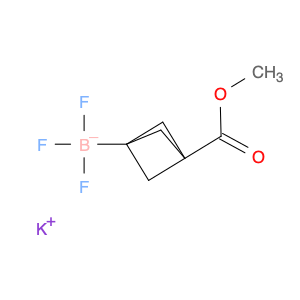
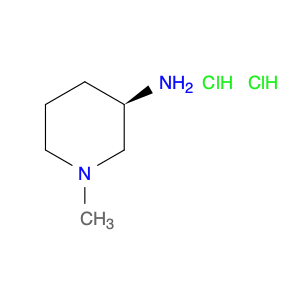
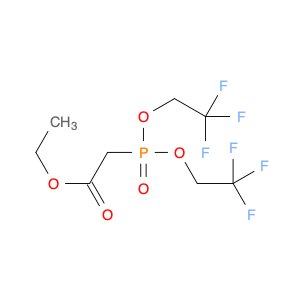
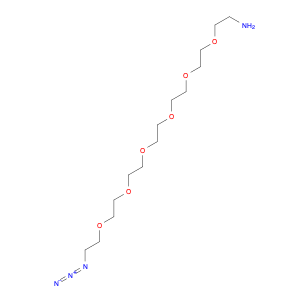
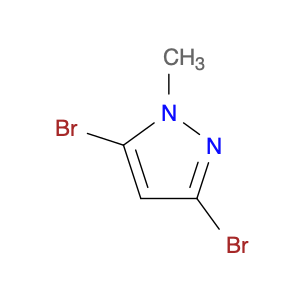
Pseudoginsenoside F11, a structurally unique compound derived from ginsenosides found in Panax ginseng, has garnered significant interest in the field of chemical synthesis. With its distinct chemical properties, Pseudoginsenoside F11 is widely utilized as a versatile building block in the synthesis of various organic molecules. Its functional groups and reactive sites enable facile modification and transformation, making it an ideal precursor for the construction of complex molecular structures.In chemical synthesis, Pseudoginsenoside F11 serves as a valuable starting material for the preparation of novel pharmaceuticals, natural product analogs, and biologically active compounds. Its ability to undergo selective functionalization reactions allows chemists to introduce specific chemical functionalities and stereochemical elements into target molecules with precision. By incorporating Pseudoginsenoside F11 into synthetic pathways, researchers can access diverse chemical space and explore new avenues for drug discovery and development.Furthermore, Pseudoginsenoside F11's unique stereochemistry and molecular architecture offer opportunities for the design and construction of chiral compounds with potential pharmacological applications. Through strategic manipulation of its stereochemical centers, chemists can access enantiopure derivatives and stereoisomeric forms, which may exhibit enhanced biological activity or specificity. This capability makes Pseudoginsenoside F11 a valuable scaffold for the synthesis of enantioenriched molecules and the study of structure-activity relationships in drug design.Overall, the application of Pseudoginsenoside F11 in chemical synthesis showcases its versatility and utility as a key building block for the creation of diverse organic compounds with therapeutic potential and structural complexity. Its role in driving innovation and discovery in the field of medicinal chemistry highlights its importance as a valuable tool for advancing scientific research and expanding the frontiers of chemical synthesis.
 sales@aaronchem.com
sales@aaronchem.com