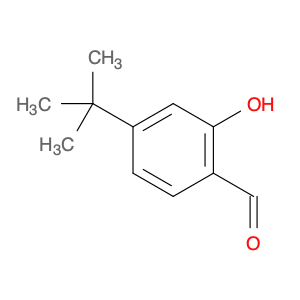
4-(tert-Butyl)-2-hydroxybenzaldehyde, also known as t-Butyl salicylaldehyde, is a versatile compound widely used in chemical synthesis for various applications. This aromatic aldehyde features a tert-butyl group and a hydroxyl group, making it a valuable building block in organic chemistry.In chemical synthesis, 4-(tert-Butyl)-2-hydroxybenzaldehyde serves as a key intermediate in the preparation of diverse organic compounds. Its unique structure and reactivity allow it to participate in a range of reactions, including condensation, oxidation, and nucleophilic addition. This aldehyde can undergo reactions with various nucleophiles, such as amines and hydrazines, to form Schiff bases and hydrazones, respectively.One important application of 4-(tert-Butyl)-2-hydroxybenzaldehyde is in the synthesis of specialized ligands for coordination chemistry. By functionalizing the aldehyde group or modifying the aromatic ring, chemists can tailor the properties of the ligands for specific metal ions or catalytic applications. These ligands play a crucial role in metal coordination complexes and catalytic reactions, offering selectivity and control over the reaction pathways.Additionally, t-Butyl salicylaldehyde is utilized in the preparation of pharmaceutical intermediates and natural product synthesis. Its presence in the molecular structure imparts specific properties to the final compounds, influencing their biological activity and pharmacological profile. By incorporating this aldehyde into the synthesis of complex molecules, chemists can access new chemical entities with potential therapeutic applications.Overall, the diverse applications of 4-(tert-Butyl)-2-hydroxybenzaldehyde in chemical synthesis highlight its significance as a versatile building block in organic chemistry. With its reactivity and functional groups, this compound offers opportunities for innovation and creativity in the design and development of novel molecules for various industries.
 sales@aaronchem.com
sales@aaronchem.com