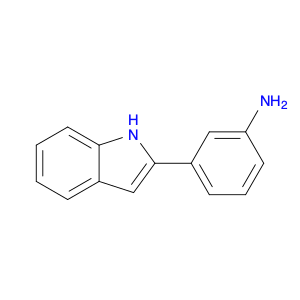
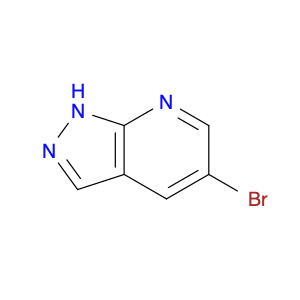
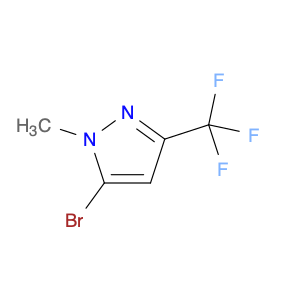
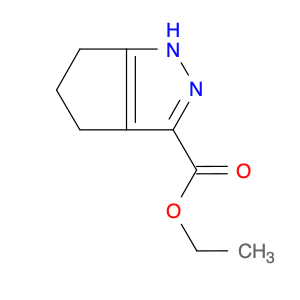
3-(1H-Indol-2-yl)benzenamine, also known as indole-2-phenylamine, is a versatile compound commonly used in chemical synthesis for various applications. In organic chemistry, this compound serves as a building block for the synthesis of biologically active molecules, pharmaceuticals, and agrochemicals. Its unique structure containing both an indole and a benzene ring allows for the functionalization and modification of the molecule to create new derivatives with diverse properties.One of the key applications of 3-(1H-Indol-2-yl)benzenamine is in the synthesis of indole-based heterocyclic compounds. By reacting this compound with different reagents and catalysts, chemists can create a wide range of indole derivatives with varying substituents and functional groups. These indole derivatives have been found to exhibit potent pharmacological activities, making them valuable in drug discovery and development.Additionally, 3-(1H-Indol-2-yl)benzenamine can be used in the development of organic materials and dyes due to its unique optical properties. By incorporating this compound into the molecular structure of polymers or dyes, researchers can tailor their properties such as absorption spectra, fluorescence, and conductivity for specific applications in optoelectronic devices, sensors, and materials science.Furthermore, the presence of an amino group in 3-(1H-Indol-2-yl)benzenamine allows for further derivatization through various chemical reactions such as acylation, alkylation, and Suzuki couplings. This enables the synthesis of more complex molecules and polymers with enhanced properties and functionalities for industrial and research purposes.Overall, the versatility and reactivity of 3-(1H-Indol-2-yl)benzenamine make it a valuable building block in chemical synthesis, offering opportunities for the development of novel compounds with diverse applications in organic chemistry, material science, and pharmaceutical research.
 sales@aaronchem.com
sales@aaronchem.com