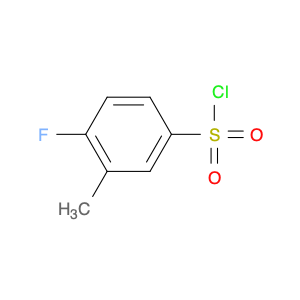
The 4-Fluoro-3-methylbenzenesulfonyl chloride, also known as $name$, is a versatile chemical reagent commonly used in the field of chemical synthesis. Due to its unique structure and properties, it plays a crucial role in various organic transformations and reactions.One of the key applications of $name$ in chemical synthesis is its utility as a sulfonyl chloride compound. Sulfonyl chlorides are valuable intermediates in the synthesis of pharmaceuticals, agrochemicals, and functional materials. With its specific fluoro and methyl substituents, 4-Fluoro-3-methylbenzenesulfonyl chloride offers distinct reactivity profiles compared to other sulfonyl chlorides, allowing for selective modifications and derivatization of organic molecules.Additionally, 4-Fluoro-3-methylbenzenesulfonyl chloride is often employed as a cross-coupling reagent in the formation of carbon-carbon and carbon-heteroatom bonds. Its ability to introduce the sulfonyl group into target molecules under mild reaction conditions makes it a valuable tool for the synthesis of complex organic compounds with improved properties.Furthermore, $name$ can serve as a building block in the preparation of sulfonamide derivatives, which are prevalent in medicinal chemistry and serve as key structural motifs in drug design. By incorporating the fluoro and methyl functionalities, specific interactions and properties can be imparted to the final molecules, enhancing their biological activity and pharmacokinetic profiles.Overall, the unique reactivity and versatility of 4-Fluoro-3-methylbenzenesulfonyl chloride make it a valuable reagent in chemical synthesis, enabling the efficient and tailored synthesis of diverse organic compounds with potential applications in various industries.
 sales@aaronchem.com
sales@aaronchem.com