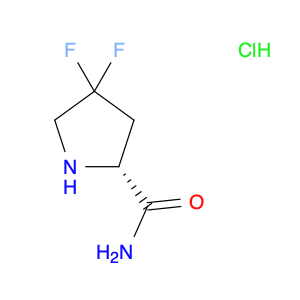
Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
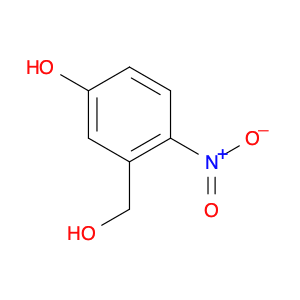
5-Hydroxy-2-nitrobenzyl alcohol
Catalog#: AR00EB1J | CAS#: 60463-12-9 | MDL#: MFCD00134308 | MF: C7H7NO4 | MW: 169.1348
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 100mg | $5.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $5.00 | Global Stock | Buy Now | Add To Cart | ||
| 1g | $19.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $82.00 | Global Stock | Buy Now | Add To Cart | ||
| 25g | $393.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR00EB1J |
| Chemical Name | 5-Hydroxy-2-nitrobenzyl alcohol |
| CAS Number | 60463-12-9 |
| Molecular Formula | C7H7NO4 |
| Molecular Weight | 169.1348 |
| MDL Number | MFCD00134308 |
| SMILES | OCc1cc(O)ccc1[N+](=O)[O-] |
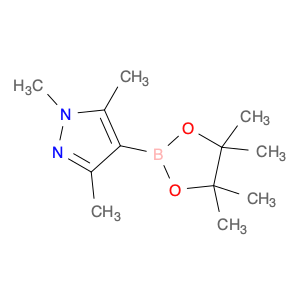
1,3,5-TRIMETHYL-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-1H-PYRAZOLE
844891-04-9
| GHS Pictogram |

|
| Signal Word | Warning |
| UN# | N/A |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305+P351+P338 |
| Class | N/A |
| Packing Group | N/A |