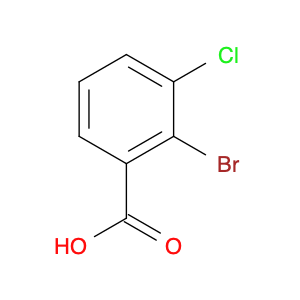
2-Bromo-3-chlorobenzoic acid is a versatile chemical compound commonly utilized in chemical synthesis processes. This specific compound plays a crucial role in various reactions due to its unique properties and reactivity. In the realm of organic chemistry, 2-Bromo-3-chlorobenzoic acid is frequently employed as a key intermediate in the synthesis of pharmaceuticals, agrochemicals, and other fine chemicals.The presence of both bromine and chlorine functional groups on the benzene ring of the molecule enables diverse transformations and functionalization. One of the prominent applications of 2-Bromo-3-chlorobenzoic acid is its involvement in the formation of biaryl compounds, which are essential structural motifs in many natural products and bioactive molecules. Additionally, this compound serves as a building block in the preparation of heterocyclic compounds and complex organic frameworks.By judiciously manipulating the reactivity of 2-Bromo-3-chlorobenzoic acid through various chemical reactions such as Suzuki coupling, Grignard reactions, and Heck reactions, chemists can access a broad array of structurally diverse compounds with tailored properties. The strategic incorporation of this compound into synthesis pathways empowers chemists to construct intricate molecular architectures efficiently and with high selectivity. The versatility and utility of 2-Bromo-3-chlorobenzoic acid make it a valuable asset in the toolbox of synthetic chemists striving to create novel molecules for pharmaceutical, agricultural, and material science applications.
 sales@aaronchem.com
sales@aaronchem.com