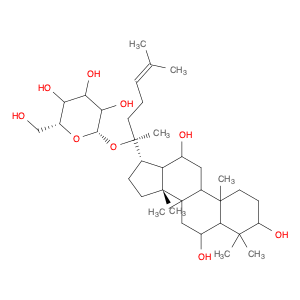
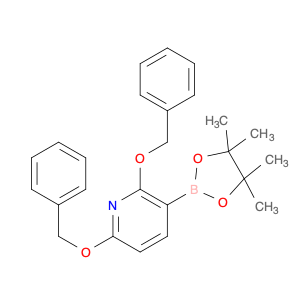
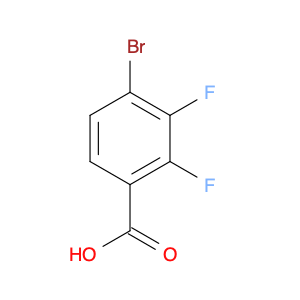
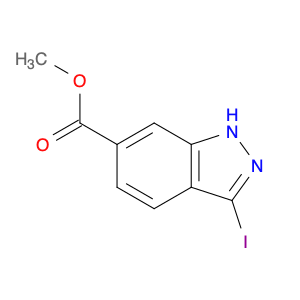
Ginsenoside F1, a natural compound found in Panax ginseng, has gained significant attention for its unique properties in chemical synthesis. This bioactive molecule has been utilized in the field of organic chemistry as a valuable starting material for the synthesis of various complex compounds. Due to its specific structural features, Ginsenoside F1 serves as an excellent precursor in the development of novel pharmaceuticals, agrochemicals, and materials with potential applications in medicine and industry.One of the key applications of Ginsenoside F1 in chemical synthesis is its role as a versatile building block for the production of bioactive derivatives. Through strategic modifications and functional group transformations, chemists can harness the structural diversity of Ginsenoside F1 to generate a wide range of analogs with enhanced biological activities. These modified compounds have shown promising results in the development of new drug candidates, providing potential treatments for various diseases and ailments.Furthermore, Ginsenoside F1 has also been utilized as a chiral auxiliary in asymmetric synthesis, enabling the production of enantiomerically pure compounds with high stereoselectivity. By leveraging the chiral nature of Ginsenoside F1, chemists can efficiently access optically active molecules that are crucial in the pharmaceutical and agrochemical industries. This approach not only streamlines the synthesis process but also offers greener and more sustainable routes to important chemical entities.In summary, Ginsenoside F1 plays a vital role in chemical synthesis by serving as a valuable resource for the creation of complex molecules with diverse applications. Its versatile nature and unique structural characteristics make it a valuable asset in the development of novel compounds with potential therapeutic and industrial significance.
 sales@aaronchem.com
sales@aaronchem.com