Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
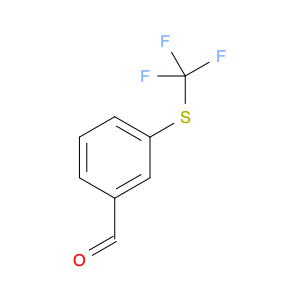
3-(TRIFLUOROMETHYLTHIO)BENZALDEHYDE
Catalog#: AR00DD9E | CAS#: 51748-27-7 | MDL#: MFCD00236335 | MF: C8H5F3OS | MW: 206.1849
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 100mg | $4.00 | Global Stock | Buy Now | Add To Cart | ||
| 250mg | $9.00 | Global Stock | Buy Now | Add To Cart | ||
| 1g | $21.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $100.00 | Global Stock | Buy Now | Add To Cart | ||
| 10g | $199.00 | Global Stock | Buy Now | Add To Cart | ||
| 25g | $380.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR00DD9E |
| Chemical Name | 3-(TRIFLUOROMETHYLTHIO)BENZALDEHYDE |
| CAS Number | 51748-27-7 |
| Molecular Formula | C8H5F3OS |
| Molecular Weight | 206.1849 |
| MDL Number | MFCD00236335 |
| SMILES | O=Cc1cccc(c1)SC(F)(F)F |
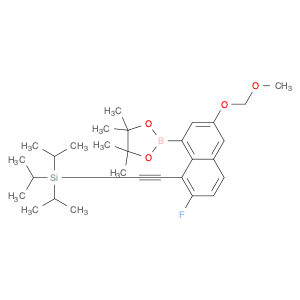
1,3,2-Dioxaborolane, 2-[7-fluoro-3-(methoxymethoxy)-8-[2-[tris(1-methylethyl)silyl]ethynyl]-1-naphthalenyl]-4,4,5,5-tetramethyl-
2621932-37-2
| GHS Pictogram |

|
| Signal Word | Warning |
| UN# | N/A |
| Hazard Statements | H227-H302-H315-H319-H335 |
| Precautionary Statements | P261-P305+P351+P338 |
| Class | N/A |
| Packing Group | N/A |