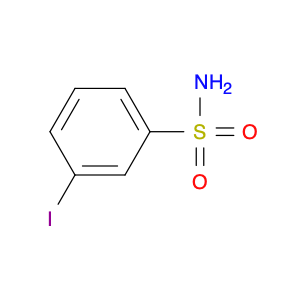
3-Iodobenzenesulfonamide is a versatile compound widely utilized in chemical synthesis as a key building block in various organic reactions. Its unique molecular structure, consisting of a sulfonamide group attached to an iodobenzene ring, offers distinct reactivity and functionality that make it valuable in the creation of complex molecules.One notable application of 3-Iodobenzenesulfonamide is in the synthesis of sulfonamides, which are important pharmaceutical compounds with diverse biological activities. By utilizing this compound as a starting material, chemists can introduce the sulfonamide functionality into target molecules, thereby modulating their pharmacological properties.Additionally, 3-Iodobenzenesulfonamide is commonly employed in transition metal-catalyzed cross-coupling reactions to form C–N bonds. This type of reaction enables the efficient construction of biaryl compounds, which are prevalent in medicinal chemistry and materials science. The iodine atom in 3-Iodobenzenesulfonamide serves as a handle for further functionalization, allowing for the introduction of various substituents and the synthesis of diverse chemical structures.Furthermore, 3-Iodobenzenesulfonamide can participate in electrophilic aromatic substitution reactions, expanding its utility in the synthesis of aryl sulfonamides and related compounds. Its ability to undergo multiple modes of reactivity makes it a valuable reagent for chemists seeking to access novel molecules for biological evaluation or material design.
 sales@aaronchem.com
sales@aaronchem.com