Login |
Create New Account
 sales@aaronchem.com
sales@aaronchem.com
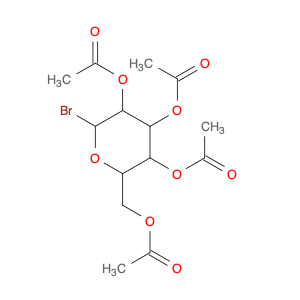
a-D-Galactopyranosyl bromide, tetraacetate
Catalog#: AR00I6GH | CAS#: 3068-32-4 | MDL#: MFCD00063686 | MF: C14H19BrO9 | MW: 411.1993
| Packsize | Purity | Price | Availability | Quantity | ||
|---|---|---|---|---|---|---|
| 1g | $3.00 | Global Stock | Buy Now | Add To Cart | ||
| 5g | $5.00 | Global Stock | Buy Now | Add To Cart | ||
| 10g | $10.00 | Global Stock | Buy Now | Add To Cart | ||
| 25g | $24.00 | Global Stock | Buy Now | Add To Cart | ||
| 100g | $93.00 | Global Stock | Buy Now | Add To Cart |
- Description
- Application
- Related Products
- Featured Products
- Safety Information
| Catalog Number | AR00I6GH |
| Chemical Name | a-D-Galactopyranosyl bromide, tetraacetate |
| CAS Number | 3068-32-4 |
| Molecular Formula | C14H19BrO9 |
| Molecular Weight | 411.1993 |
| MDL Number | MFCD00063686 |
| SMILES | CC(=O)OC[C@H]1O[C@H](Br)[C@@H]([C@H]([C@H]1OC(=O)C)OC(=O)C)OC(=O)C |
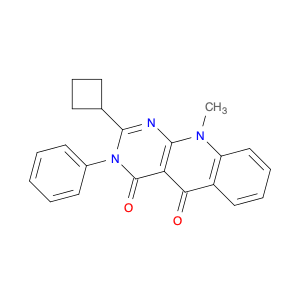
2-Cyclobutyl-10-methyl-3-phenylpyrimido[4,5-b]quinoline-4,5(3H,10H)-dione
1613509-49-1
| GHS Pictogram |

|
| Signal Word | Warning |
| UN# | N/A |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305+P351+P338 |
| Class | N/A |
| Packing Group | N/A |